Твои современные решения для ванной комнаты
Узнаете с чего необходимо начинать ремонт в ванной комнате. Самые распространенные ошибки, которые допускаются в ремонте ванной. Советы по планировке и отделке ванной комнаты
Сможете вдохновиться самыми красивыми ванными комнатами в различных стилях, идеями декора и оформления ванной комнаты. Получить советы от профессионалов по оформлению ванной комнаты
Все что необходимо знать о ремонтных работах в квартире, доме своими руками: выбор материалов, инструментов. Отделочные работы. Современные технологии проведения ремонтных работ. Советы специалистов по замене и установке сантехники
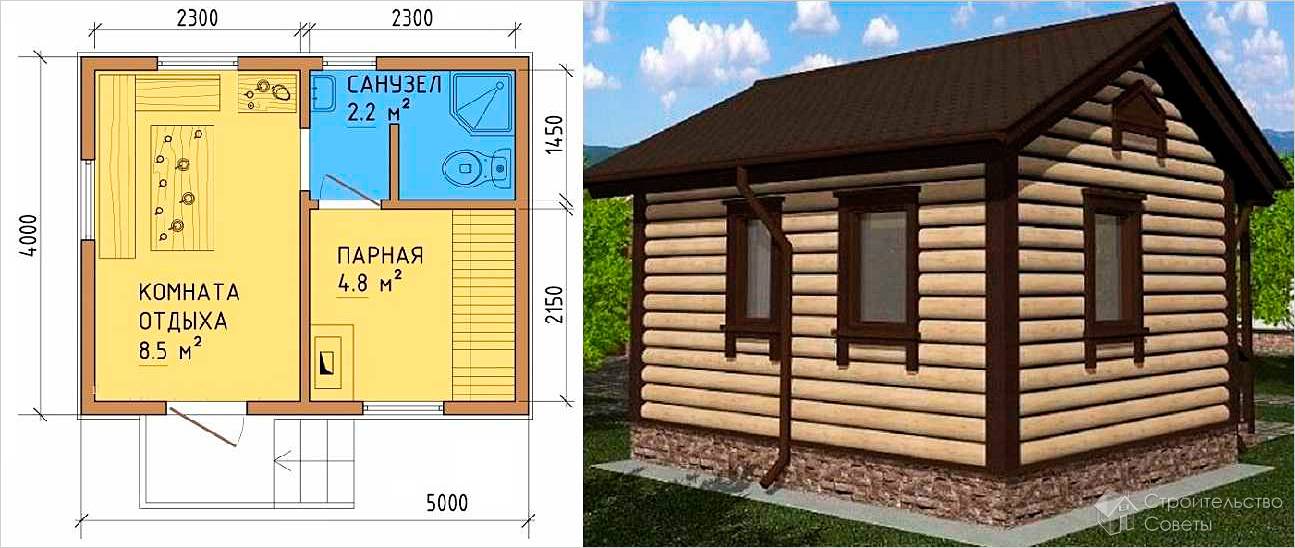
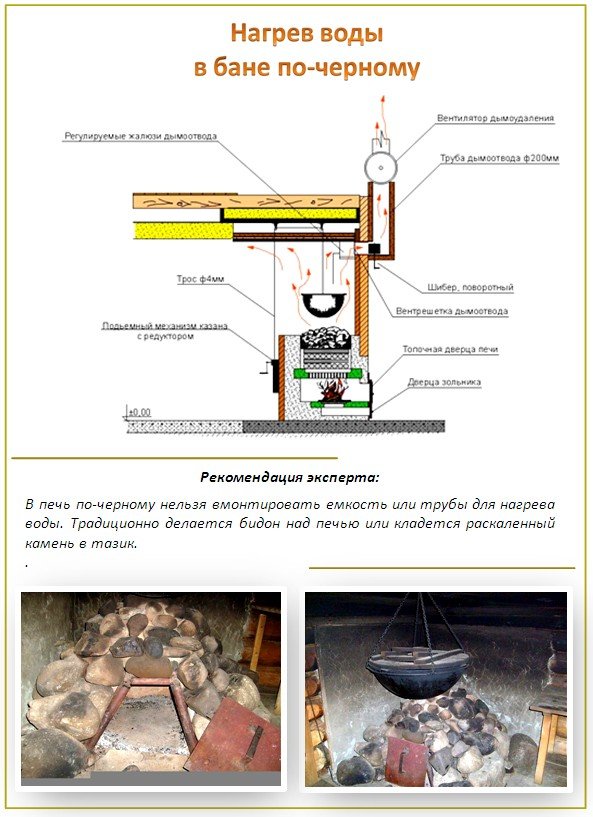
Узнаете, что необходимо учитывать при строительстве своими руками. Виды строительных работ. Кладка стен. Перекрытие крыши. Инструкция как правильно сделать заливной пол в помещении